Innovation: Diagnostics
Targeted Cancer(s): Skin cancers
Leadership: Stephen Stetson
Stage of Business: Early stage

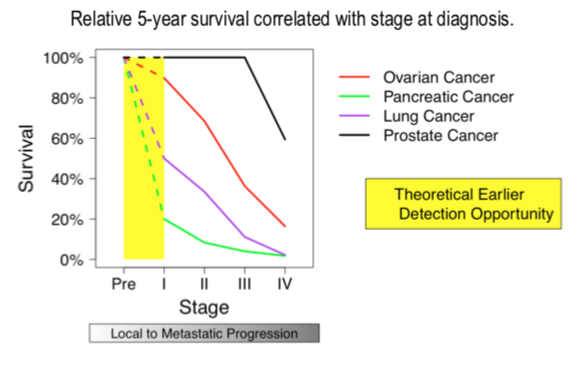
EARLY DETECTION OF CANCER SAVES LIVES.
The survival rate for people whose cancer is detected early is much better than that for later stage cancers – no matter what type.
Tens of billions of dollars are spent each year on R&D for cancer treatment. Less than one percent of the funding is spent on early detection. Yet, early detection will save more lives than any form of late stage treatment.
The National Foundation for Cancer Research (NFCR) is supporting a new solution for early cancer detection through AIM-HI. The break-through technology offers great promise for safe and non-invasive screening for many types of cancer.
One of the newest and most promising methods for early cancer detection is known as an Optical Biopsy – the use of light waves to detect and differentiate cancer cells from normal cells. Optical biopsies offer low-cost, safe and accurate methods to detect many forms of cancer at the earliest stage possible.
Optical biopsies provide the ability to screen more people and more sites per person, faster and cheaper than other methods. Portable and low-cost optical sensors will make it possible to provide cancer screening services to underserved and disadvantaged populations worldwide, reducing the burden of cancer on all of society.
Optical biopsies have the potential to transform how we detect and treat cancer. Early detection can change a diagnosis of cancer from a life-threatening disease to a short-term treatment with full recovery.
The best way to improve survival rates is through early detection and intervention.
The majority of cancer research focuses on drug development. Less than 1% of translational funding goes to early detection even though early detection and treatment is far more effective than that for later stages.
“The greatest advances in cancer research will be obtained — and more lives saved — by focusing translational research on early detection.”
(Source: Canary Center at Stanford for Cancer Early Detection)
(Source: National Cancer Institute)
LightWave Diagnostics’ Optical Biopsy solution involves two components:
A.) an electro-optical sensor for measuring electromagnetic waves from cellular metabolism, and
B.) a Cloud-based Artificial Intelligence (AI) and Machine Learning (ML) data analytics platform for diagnostic analysis of the cellular data.
LightWave Diagnostics’ intellectual property (IP) includes, both, hardware and software innovation for unprecedented analysis of cellular metabolism in real time.
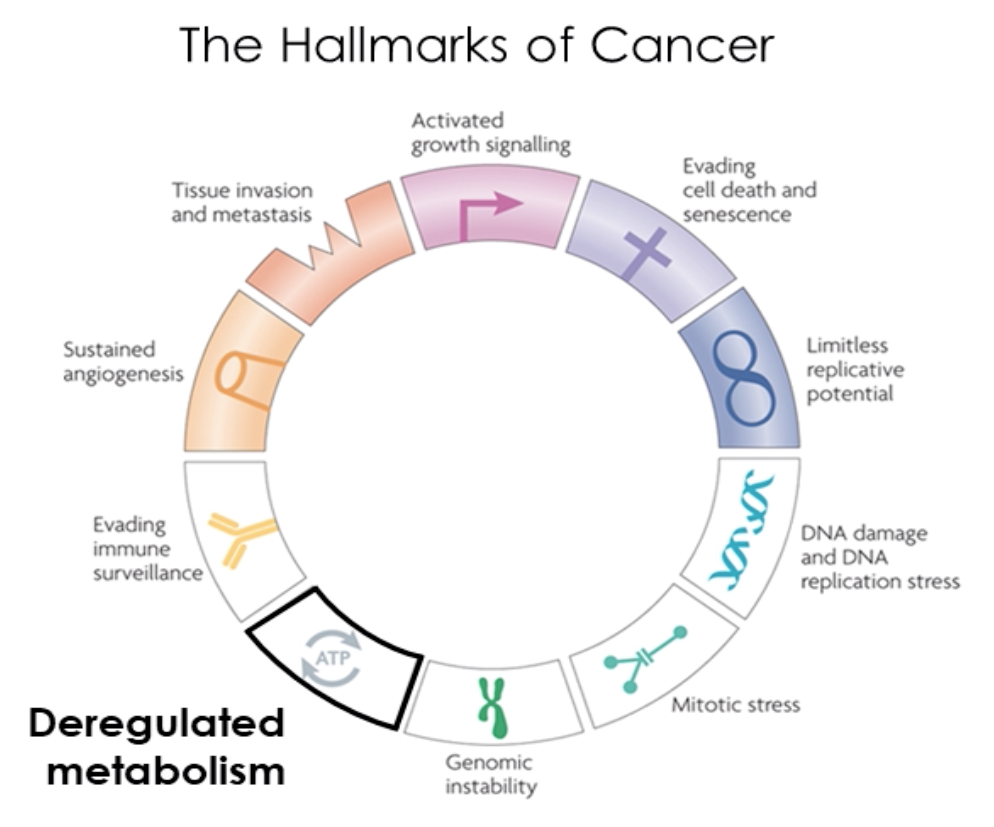
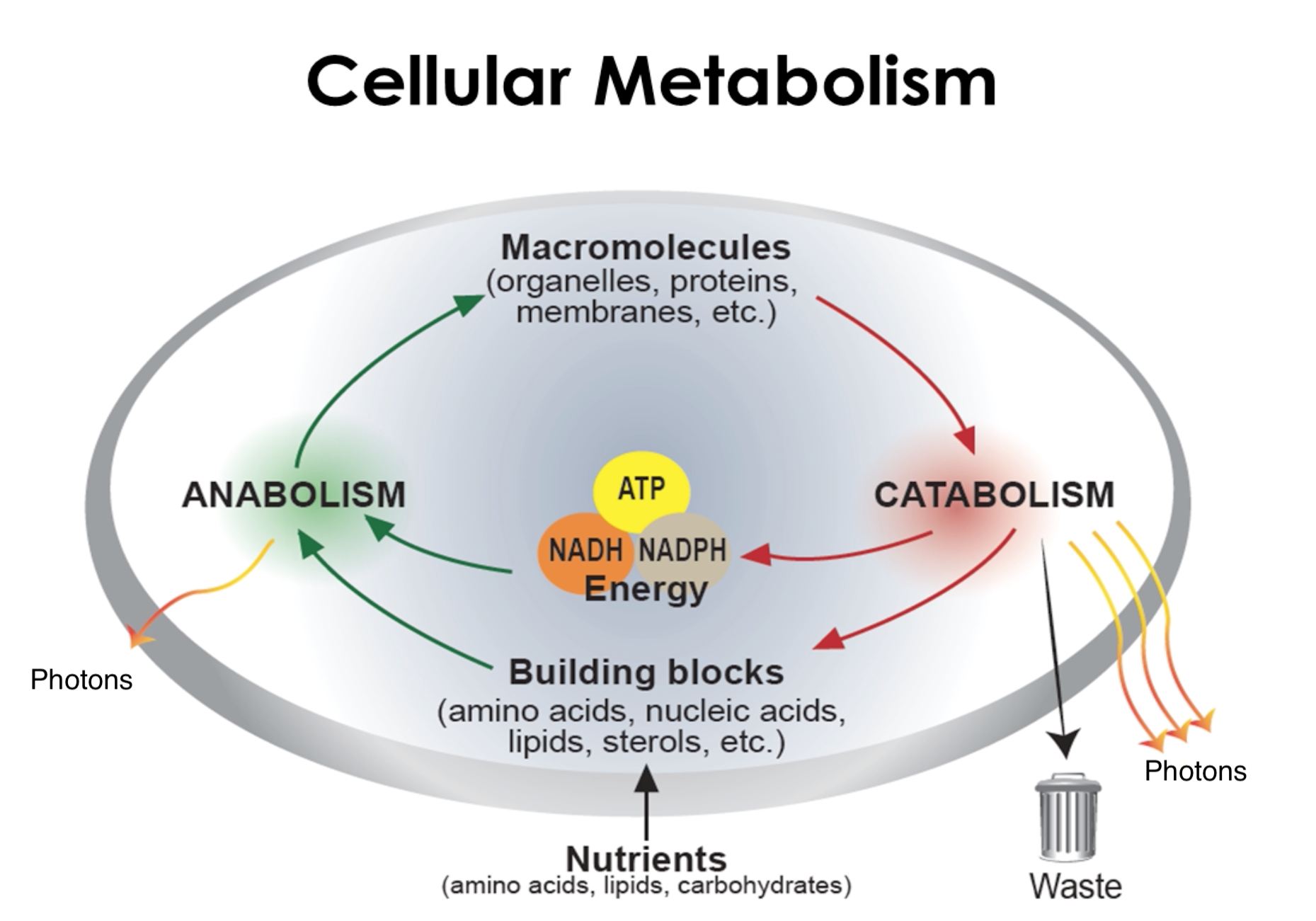
One of the Hallmark characteristics of all cancers is the change in cellular metabolism.
The deregulated metabolism emits energy at a different intensity than normal cells, which the optical sensor can measure.
The optical sensor will collect digital measurements and upload the data to the diagnostic service in the Cloud. The diagnostic procedure will use AI/ML-based algorithms to analyze the data and compare it to the central database of cellular profiles. Together, the portable optical device for data collection and the remote diagnostic service will form a platform for easy deployment of the technology in a wide range of settings worldwide.
While the technology is applicable to all forms of cancer, the initial application will be for skin cancer and other cancers near the surface of the body. The solution will provide a new method for cancer screening that eliminates the need for surgical biopsy and the time and expense of pathology lab examination. No more cutting, scarring, risk of infection or waiting for pathology lab results. The measurement is made in seconds, and the diagnosis is ready in minutes.
The USFDA has already issued a letter of risk determination categorizing LightWave Diagnostics’ proposed study as a nonsignificant risk (NSR) device study, which allows the technology to proceed to clinical trials without the need for an Investigational Device Exemption and in-depth safety review by an Institutional Review Board.
Digital pathology and remote diagnostics for medical imaging technology are already used around the world. LightWave Diagnostics’ solution will advance the state of this technology a step further by making cancer detection fully digital, from data acquisition to diagnostic analysis, eliminating the need for subjective and manual examination of extracted tissue.
As the first optical biopsy technology under the AIM-HI program LightWave Diagnostics’ solution will complement all of the other AIM-HI initiatives for cancer detection and intervention.
To learn more about LightWave Diagnostics, click here to visit their company website.

