Innovation: Drug discovery – small-molecule inhibitors of invasion and metastasis focusing on a specific cancer-promoting protein, MDA-9/Syntenin
Targeted Cancer(s): Applicable to the majority of metastatic and invasive cancers
Leadership: Paul. B. Fisher, M.Ph., Ph.D., President & CEO; Webster Cavenee, Ph.D.
Stage of Business: Matching Fund Application in Progress

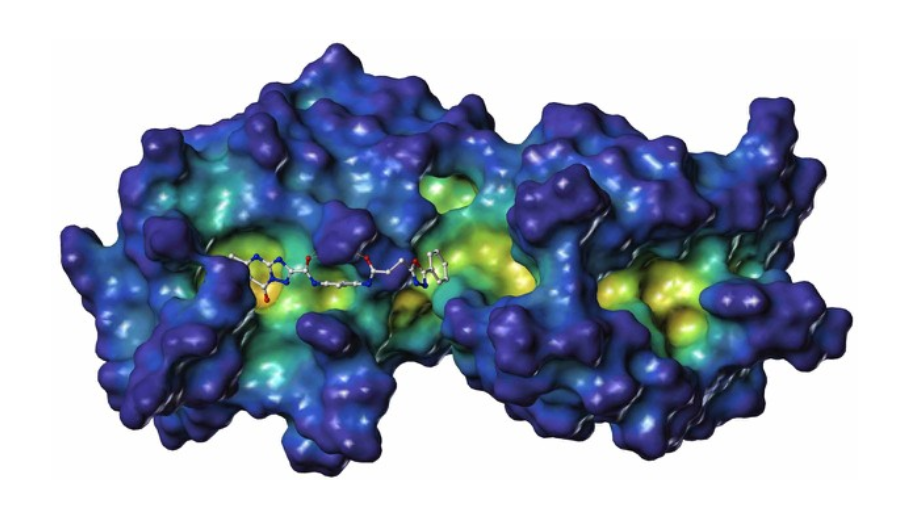
PDZ1i is a small molecule developed by InVaMet Therapeutics (IVMT) through NMR-guided fragment-based drug design that is an effective inhibitor of the ultimate and most lethal stage of cancer progression, metastasis. This novel first-in-class small molecule inhibitor binds specifically to the PDZ1 domain of the pro-metastatic gene, MDA-9/Syntenin, blocking essential protein-protein interactions regulating essential cancer signaling properties. Based on the relevance of MDA-9/Syntenin to cancer development, metastasis, and patient-survival, as well as the broad-spectrum anti-cancer activity of the PDZ1i drug, bringing this lead agent into the clinic is a primary objective of IVMT.