InterLeukin Combinatorial Therapies, Inc
InterLeukin Combinatorial Therapies, Inc

InterLeukin Combinatorial Therapies, Inc
Company Overview
Innovation: Therapeutics & drug discovery – Cancer Terminator Viruses for the targeted therapy of a diverse array of aggressive tumor types
Targeted Cancer(s): GBM Melanoma
Leadership: Paul. B. Fisher, M.Ph., Ph.D., President & CEO; Webster Cavenee, Ph.D.
Stage of Business: Approved for Matching Funds from VBHRC VA Catalyst Program leading to Phase I Clinical Trials
Opportunity
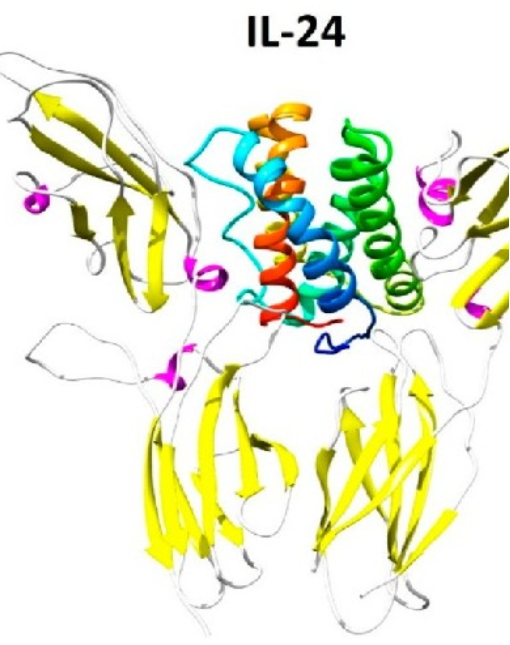
IL-24 (mda-7), and its next generation “Superkine”, are novel cytokines developed and being optimized by Interleukin Combinatorial Therapies (ILCT). Through genetic engineering the next generation of cancer-selective interleukin (cytokine)-based therapeutics; secreted proteins that can locate and destroy tumor cells in all parts of the body by directly promoting programmed and immunogenic cell death, inhibiting new blood vessel formation (angiogenesis) and sensitizing tumor cells to conventional therapies (including radiation, chemotherapy and immunotherapy), without inducing toxicity in normal cells or tissues. Through a process called “bystander antitumor activity” our unique engineered therapeutic interleukins (cytokines), ETCs (Enhanced Therapeutic Cytokines; NG.IL-24; Superkine), destabilize cancer cells resulting in direct killing or indirect toxicity through activation of the immune system and inhibition of angiogenesis.
The ETCs can be administered through vector-mediated delivery strategies (such as viruses), cellular-delivery (by means of T cells) or as pure therapeutic proteins. In addition, we have developed an innovative method, ultrasound-targeted microbubble-destruction (UTMD), to employ a ‘stealth’ delivery strategy permitting focused-targeting of therapeutics directly to tumors or the tumor microenvironment. Remarkably, the therapeutic efficacy of the cytokines against primary tumors and metastases (including bone metastases) can be enhanced further by combining them with radiation, chemotherapy or rationally-designed anti-cancer targeted drugs.
Unmet Medical Need
- It is estimated that ~90% of patients with advanced cancers die as a result of complications associated with tumor cell invasion into normal tissue and metastatic spread from primary tumor sites to secondary locations in the body, including lung, liver, bone, brain and other organs.
- Glioblastoma (GBM), the first disease ILCT will target, is the most common and aggressive type of brain cancer and whose prognosis is extremely poor, despite a limited number of new therapies approved over the last 10 years. This is because GBM cells are genetically heterogeneous, that delivery of agents which require engaging most or all tumor cells is difficult and that GBM cells migrate into the brain parenchyma, making complete surgical resection difficult and virtually assuring recurrence. Median overall survival for GBM is approximately 15 months and the average five-year survival rate is less than five percent.
- It is estimated that ~90% of patients with advanced cancers die as a result of complications associated with tumor cell invasion into normal tissue and metastatic spread from primary tumor sites to secondary locations in the body, including lung, liver, bone, brain and other organs.
Differentiation
- Targeting cancer cells with selectively replicating viruses such as Ad.5/3-CTV to cause cancer cells to produce and secrete IL-24 and create a broader field effect, should have effects on both primary site tumors and more distant tumors. In experimental animals this leads to longer survival, even with animals that have distant metastases. This portends longer times to recurrence and longer survival for GBM patients.
- Since IL-24 can be delivered in a variety of ways, this is likely to provide even broader applicability. Experimental data are in hand showing efficacy of IL-24 in cancers, including GBM, melanoma and carcinomas of the breast, colon, lung, bladder, liver, pancreas, prostate.
- Combining IL-24 with checkpoint inhibitors, chemotherapy and/or radiation therapy, anticancer monoclonal antibodies to enhance antibody-dependent cellular cytotoxicity (ADCC) of these antibodies, antibody cytokine fusion proteins, and anti-CD40 to facilitate tumor-specific responses.
Asset Profile & Development Plan
- Potency and Duration of Action: potent anti-cancer and anti-metastatic effects of IL-24 in several animal models promoting long-term survival.
- Proof-of-concept: IL-24 is efficacious in several rodent models of GBM, and the other cancers mentioned above that employ xenotransplantation of human tumor tissues as well as in spontaneous models driven by genetic modification.
- Safety and Tolerability: IND enabling toxicology has been discussed with the FDA. Given that the early version of IL-24 has been administered to patients and has shown no adverse events and displays clinical efficacy, it is unlikely that any issues will be uncovered in Phase 1 clinical testing of the newer version ETC (Superkine).
- Clinical development plan: the initial clinical trial will test safety in GBM patients treated with IL-24-CTV. This trial should initiate within a year of funding.


