Arbele
Arbele

Arbele
Company Overview
Innovation: Targeting Immune Engagement
Targeted Cancer(s): Gastrointestinal cancers, including pancreas, stomach, liver and bowel
Scientific Leadership/Leadership: Prof. John Luk, DMedSc., EMBA, CEO and Founder; Dr. Dennis Wong, MD, Global Clinical Officer; Mr. David Lam, Head of Corporate Finance
Stage of Company: Set to initiate clinical study of our first -in-class bispecific GI Cancer targeting/T-cell engager immunotherapy, ARB 202
Opportunity
Cancers of the gastrointestinal tract are frequent in Asia; stomach and liver cancers are a leading cause of death. In the west, while early screening can detect these “internal” cancers, many are detected too late and have already spread and require systemic therapy but long-term response has been elusive. Prolonged survival has been achieved with immunotherapy for some solid tumors, but current checkpoint inhibitors lower the threshold of host immune system reactive to self as well as the cancer cells. Thus, unwanted autoimmune disease induced by the checkpoint inhibitors alleviates their effectiveness.
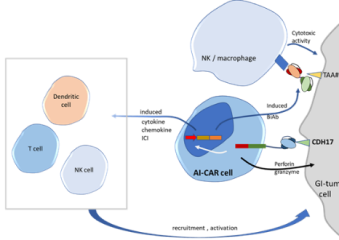
Cadherin-17 oncogene was discovered in Hong Kong by Arbele’s founder to be abnormally high on cancer cells of the gut and provide a way to target immune therapy to the cancerous cells. Arbele has developed a multi-targeting antibody platform and a complementary platform to use those proteins to modify immune cells to target abnormal cancer cells. Arbele seeks strategic partners and investors for long-term development of our lead drug candidates, ARB202, a novel CDH17/CD3 bispecific T-cell engager, and ARB001, potentially allogenic cord-blood-CAR-NK cells, to provide therapeutic options to patients suffering from GI cancer.
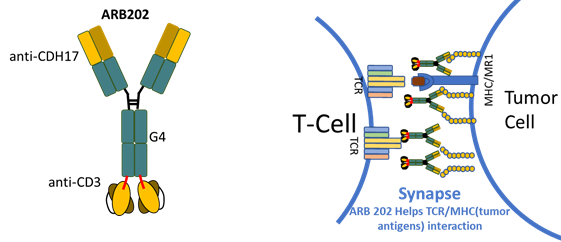
Unmet Medical Need
- Stomach and liver cancers are a leading cause of death and more prevalent in Asians.
- GI cancers if not screened and/or detected early cannot be removed by surgery having limited therapies for long term survival.
- Untargeted immunotherapies that use one’s own immune system to attack cancer are hampered by inducing uncontrolled autoimmune reactions that can be lethal.
Differentiation
- Our patented GI target cadherin-17 is aberrantly over expressed in GI cancers with limited expression in normal tissue making it a “clean” caner targeting protein.
- Our multitargeting antibodies allow us to redirect immune cells to cancerous cells and potentially allows its use in combination with checkpoint inhibitors which decreases the immune threshold more specifically at the tumor burden sites.
- Our multitargeting molecules can also be used to modify immune cells for cell-based therapy.
Asset Profile & Development Plan
- Potency: Very High affinity to GI cancer target
- Duration of Action: Targets long term immunity to treated cancer.
- Safety and Tolerability: GI cancer targeting of immune engagement expected to limit autoimmunity.
- Intellectual Property: GI cancer target Cadherin-17 discovered and patented in Hong Kong.
- Clinical Development Plan: Seamless clinical studies in targeted GI patients evaluating both safety and efficacy continuously.
- Proof of Concept: Clinical proof of concept possible early given poor prognosis in targeted GI cancers.

