Akrotome Imaging, Inc
Akrotome Imaging, Inc

Akrotome Imaging, Inc
Company Overview
Innovation: Medical Imaging – molecular imaging to guide surgeons in performing a complete resection of tumor tissues during the first operation of Breast Conservation Surgery.
Targeted Cancer(s): 80% solid tumor cancers
Leadership: Brian Straight, President & CEO; Matthew Bogyo, PhD., Co-Founder; James Basilion, PhD., Co-Founder (Click here to view our team)
Stage of Business: Ready to Apply for Clinical Trial
Opportunity
6qcICG is uniquely flexible. It can be administered in-vivo systemically, in-vivo topically, and ex-vivo topically. The probe is highly selective and remains optically silent until it encounters cancer tissue. When it detects cancer, 6qcICG robustly activates in minutes, making it ideally-suited to intraoperative cancer tissue and margin assessment.
Akrotome is in a unique position to rapidly enter the market and provide a means to significantly impact the quality of surgical resection. Nearly 4 million procedures a year could potentially benefit from this technology.
Unmet Medical Need
The best chance of a complete cure for many cancers is complete, surgical resection. The challenge for surgeons is that it is very difficult to determine where cancer ends, and healthy tissue begins. Assessment usually takes place in pathology, after the surgical procedure is complete and the patient has returned home. Failure to achieve complete resection dramatically changes the subsequent treatment of the disease, with significant negative consequences for the patient and a large, negative impact on healthcare economics.
Almost 2 million people in the US and 45 million people worldwide will be diagnosed with cancer this year. Breast cancer, colon cancer, prostate cancer, and lung cancer are the most common cancers and the largest contributors to cancer mortality. Complete resection failure for these cancers runs from 7% to 85%.
Improvements in the complete resection rates for these and other cancers would have dramatic and positive public health benefits.
Current technologies for intraoperative assessment of tumor margin infiltrates (e.g. cytology and frozen section) have significant problems and have not proven robust enough for widespread clinical adoption.
There is a clear, unmet clinical need for a technology that can rapidly, globally and accurately assess the status of tumor margins while the patient is still in the OR.
The cancer surgery market is large and growing. Annually, more than 4 million surgical procedures could benefit from an enhanced cancer margin identification technology.
First target is BCS surgery (lumpectomy). Repeat surgery rate due to incomplete resection averages 25% (around 60,000 surgeries per year). The negative economic impact on institutions alone is more than $300 million annually. Patients and clinicians recognize the unmet clinical need and are actively seeking solutions. Benefits of improvement in resection rates (reduced risks of repeat procedures, enhanced mortality/morbidity, enhanced cosmesis and quality of life for patients, improved economics for institutions) can be precisely quantified.
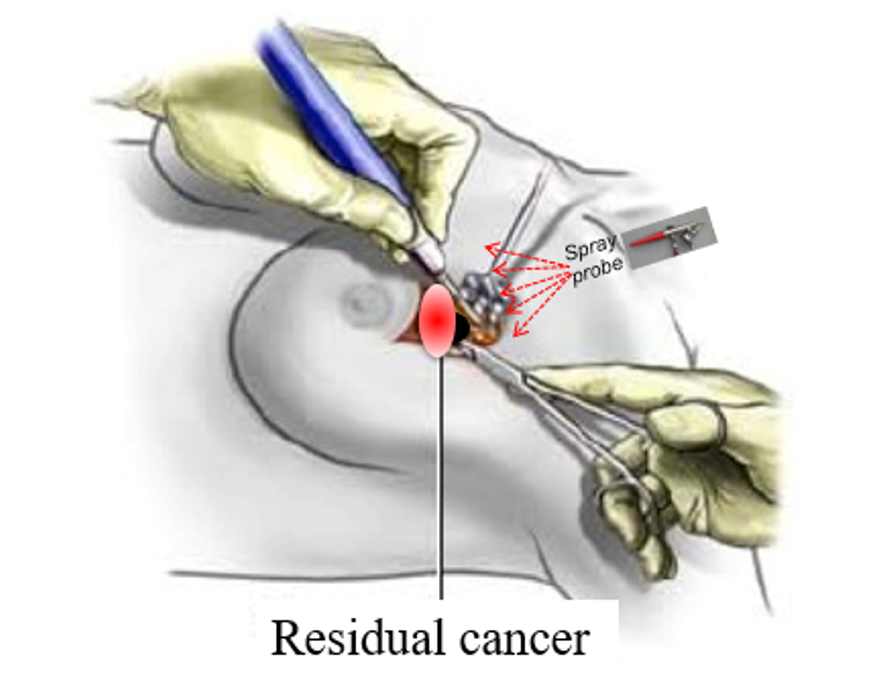
Differentiation
By targeting only those enzymes that have significantly-increased activity in breast cancer tumors (and especially in active tumor margins) 6qcICG can provide reliable intra-operative detection of residual disease after resection in breast tumor margins, enabling complete excision of diseased tissue in a single surgery.
6qcICG is a quenched probe and is activated only by cancer-associated enzymes. It is not activated by healthy tissue.
Unlike other molecular probes, which require the systemic administration of large amounts of probe hours or days before surgery, 6QcICG requires the intraoperative topical application of minute amounts of probe into the surgical cavity. The probe activates within minutes, precisely delineating any remaining cancer tissues. Even with multiple applications, total dosage will likely fall in the microdose range enabling a more direct and less expensive path to patient trials.
Asset Profile & Development Plan
Formulation Studies: phase 1 of a 2-phase project is complete and an optimal formulation of the probe has been achieved. The goal was to find a way to immobilize probe in a surgical wound with bleeding or the presence of other fluids, and on non-planar surfaces. 50 different blends of poloxamer gels were tested and the best candidate selected. In the final formulation of probe and gel, the formula is liquid at room temperature and has high viscosity at body temperature. Initial tests with in-vivo topical application in animal models show high promise with excellent probe retention and a good activation profile in the surgical cavity. In phase 2, we will compile statistically-significant sensitivity and specificity data from in vivo resections in mouse models.
Proof-of-concept: we have developed statistically-significant sensitivity and specificity data for 6qcNIR using human tumors and excised xenografts using the probe ex-vivo; these data indicate sensitivity/specificity in the 90th percentile which, if duplicated in-vivo, could reduce repeat surgery rates in BCS by up to 90%.
Next Steps: When formulation studies are complete, we plan to commence the regulatory approval process, beginning with systemic PK/TOX studies; if successful, we will move to Phase 1a acute toxicity studies (disrupted skin studies in healthy volunteers) and a small Phase 1b trial in breast cancer patients. We believe these studies can be completed in 18 months- 2 years.
Intellectual property: Akrotome owns or controls patent applications and know-how, as well as product-specific patent applications.

