SciTech Development, LLC
SciTech Development, LLC

SciTech Development, LLC
Company Overview
Innovation: Therapeutics – unique nano-delivery systems (SciTech Delivery Vehicle-SDV) to enable intravenous (IV) delivery of water-insoluble drugs
Targeted Cancer(s): non-Hodgkin Lymphoma
Leadership: Earle T. Holsapple III; Michael Burns, PhD; Ralph E. Parchment, PhD (Click here to view our team)
Stage of Business: FDA approved IND in December 2019; ST-001 clinic ready; orphan drug designation granted in 2018
Opportunity
- SciTech Development, LLC (“SCI”) is a clinical stage biopharmaceutical company that has developed unique nano-delivery systems (SciTech Delivery Vehicle-SDV) to enable intravenous (IV) delivery of water-insoluble drugs. One such challenged drug is fenretinide which has been shown in extensive clinical trials to be a safe and effective anticancer therapy. The combination of the new SDV and fenretinide has led to SCI’s first drug, ST-001.
- Fenretinide was clinically tested as a breast cancer prevention drug in more than 3,000 women and was demonstrated to be safe after long periods of use. It subsequently evolved into a cancer therapeutic and was shown to be both safe and efficacious in treating several other cancers. However, fenretinide is not currently approved as a cancer therapeutic due to the challenges of delivering adequate doses to tumor cells because the drug is hydrophobic leading to solubility problems. Unlike the earlier IV formulations, ST-001 is expected to safely deliver greater concentrations of fenretinide, thereby, prospectively achieving therapeutically effective doses without the toxic side effects observed with other delivery systems.
- Recent discovery of its immunotherapeutic effect, in which a reactivated natural immune response compliments the previously understood safe, direct, chemotherapeutic effect (two mechanisms of action), results in a unique, genetically directed process of cancer cell destruction (apoptosis).
- The market opportunity for the initially targeted diseases, T-cell non-Hodgkin lymphoma (T-cell NHL) and small cell lung cancer (SCLC), is ~ $1 billion and will likely address a broader cancer market of $5 to $25 billion including other cancers where research has demonstrated they are likely to respond to fenretinide.
Unmet Medical Need
- The treatment outcome of patients with T-cell NHL and small cell lung cancer (SCLC), SCI’s initially targeted diseases, remains dismal. Within T-cell NHL cancer indications, the cutaneous variants remain chronic but incurable. The natural disease history for many patients is marked by responses and relapses to therapies with eventual progression and fatal outcomes. For the more aggressive types of T-cell NHL, the long-term survival is approximately 20%. The 5-year overall survival rate for SCLC patients seldom exceeds 5%. Studies have demonstrated that fenretinide is a promising drug candidate for the treatment of these and other types of aggressive cancers.
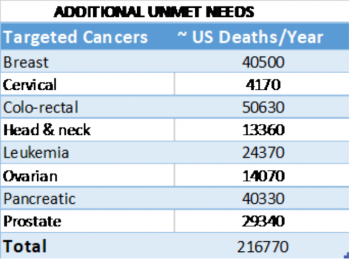
- Approximately 500,000 people per year die worldwide from diseases that preclinical and clinical data suggest will respond to treatment with fenretinide (Table 1).
Differentiation
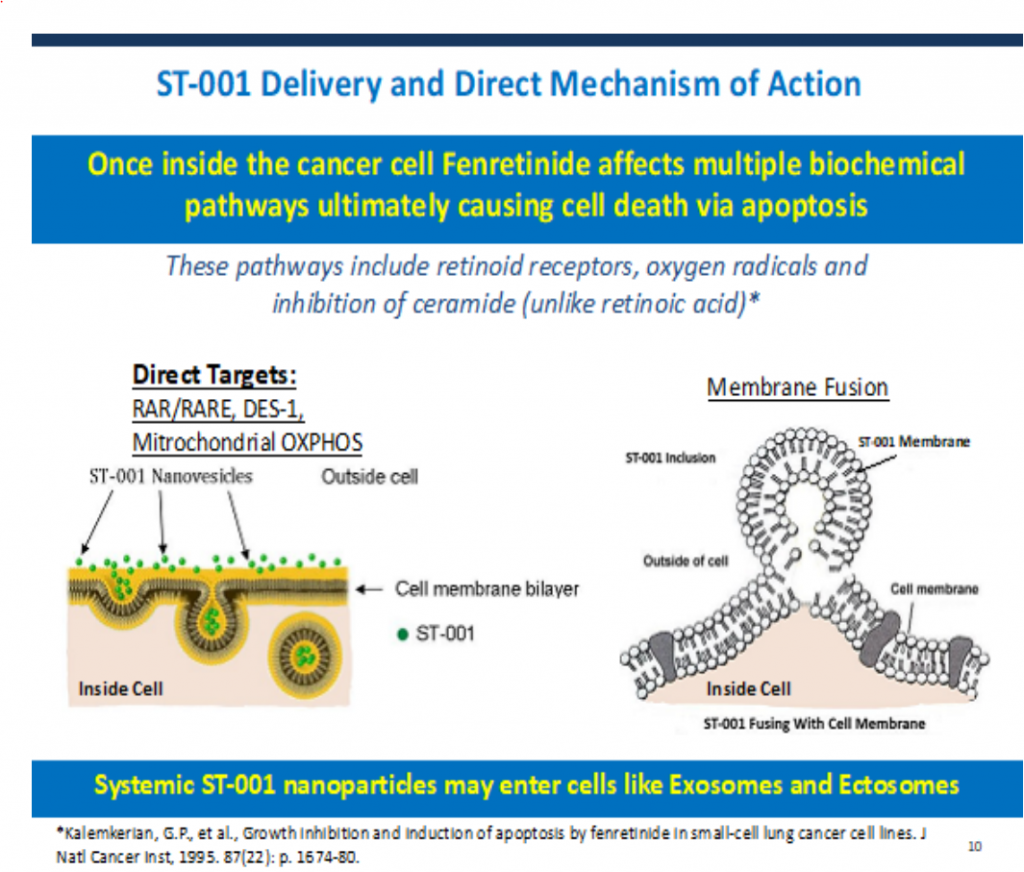
- ST-001 is a nanoparticle suspension for IV administration comprised of fenretinide in patented combination with specifically selected phospholipids (inactive ingredients). The phospholipids were chosen because they are recognized as safe for IV use in humans and because of their unique chemical and physical properties, that when combined with those of fenretinide, yielded an integrated, robust structure that contained much higher concentrations of fenretinide. Simply put, the drug is protected from water by the lipid layers of the nanoparticle.
- The lipid bilayers dissolve when the particle fuses or is absorbed by the cancer cell. Thereby, it is capable of safely delivering the range of fenretinide doses required for therapeutic efficacy – a benefit previously unattainable.
- Fenretinide is cytotoxic in various cancer cell lines, including neuroblastoma, leukemia, and lymphoma, as well as colorectal, head and neck, breast, prostate, lung, ovarian, cervical, and pancreatic cancer (Mohrbacher et al., 2007). SCI’s focus is bringing to market a single-mode drug therapy (notably, ST-001 as a standalone drug treatment). J&J has already expressed an interest in engaging in a collaboration for the treatment of small cell lung cancer.
- Managed risk is low because pre-existing data establishes fenretinide’s prior safety and efficacy which makes the case for continued safety and efficacy in its new formulation.
- Time to market is relatively short and costs are low because an accelerated/fast track clinical trial plan can be executed based on the use of prior existing data.
Asset Profile & Development Plan
- FDA regulatory status: ST-001 is clinic ready with IND# 135475; Orphan Drug Designation was granted in December 2018; accelerated clinical trial protocol approved; rapid path to market guidance** offered (<3yrs).
- Proof-of-concept: Prior clinical trial data validates the safety and efficacy of fenretinide which makes the case for its use in the new formulation and infers that the market potential is large; SCI’s clinical trial is confirmatory, not exploratory.
- Intellectual Property: SCI co-owns/licenses patents issued in the US, EU and other countries. A patent runway expansion is under development and a provisional US patent was filed in Nov. 2018. Expirations from 2030-2035.
- Clinical development plan: SCI’s immediate goal is to reconfirm fenretinide’s safety and efficacy in its new formulation, opening the potential for use in numerous other cancers.
**Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics, Guidance for Industry, FDA Draft Guidance, August 2018

