InVaMet Therapeutics
InVaMet Therapeutics

InVaMet Therapeutics
Company Overview
Innovation: Drug discovery – small-molecule inhibitors of invasion and metastasis focusing on a specific cancer-promoting protein, MDA-9/Syntenin
Targeted Cancer(s): Applicable to the majority of metastatic and invasive cancers
Leadership: Paul. B. Fisher, M.Ph., Ph.D., President & CEO; Webster Cavenee, Ph.D.
Stage of Business: Matching Fund Application in Progress
Opportunity
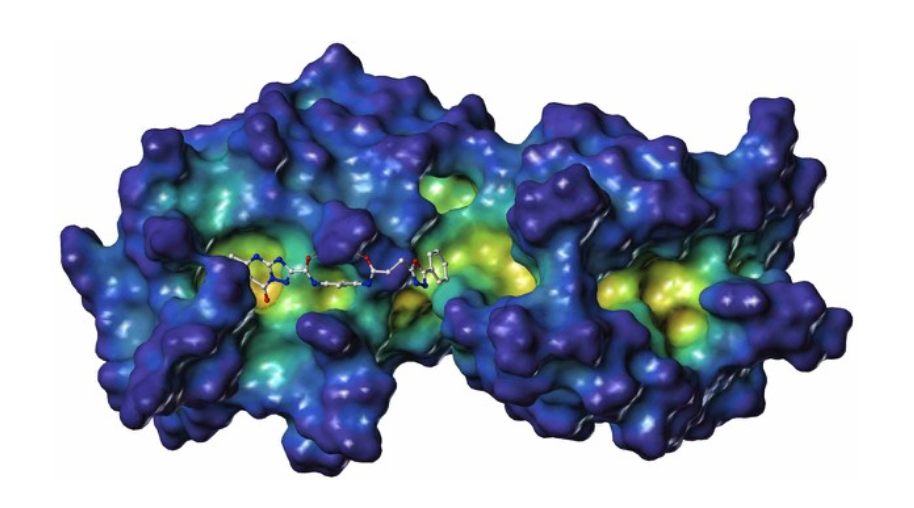
PDZ1i is a small molecule developed by InVaMet Therapeutics (IVMT) through NMR-guided fragment-based drug design that is an effective inhibitor of the ultimate and most lethal stage of cancer progression, metastasis. This novel first-in-class small molecule inhibitor binds specifically to the PDZ1 domain of the pro-metastatic gene, MDA-9/Syntenin, blocking essential protein-protein interactions regulating essential cancer signaling properties. Based on the relevance of MDA-9/Syntenin to cancer development, metastasis, and patient-survival, as well as the broad-spectrum anti-cancer activity of the PDZ1i drug, bringing this lead agent into the clinic is a primary objective of IVMT.
- PDZ1i has shown profound “anti-invasive” and “anti-metastatic” activity in multiple cancers, including melanoma, GBM, neuroblastoma and carcinomas of the breast, liver, lung, pancreas and prostate. PDZ1i inhibits retention of tumor cells in the lungs, restricts lung metastasis formation, suppresses breast and prostate metastases, blocks metastatic cancer cell attachment and dissemination and inhibits invasion, thereby prolonging survival following xenograft implantation into nude mice. Additionally, when combined with other modes of therapy, including chemotherapy, radiotherapy and immunotherapy, therapeutic outcomes are significantly enhanced.
- Although not cytotoxic to cancer or normal cells alone, when PDZ1i is combined with currently used therapeutic treatments (including chemotherapy, radiation therapy or immunotherapy), “anti-invasion therapy” is converted to “cytotoxic therapy”.
Unmet Medical Need
- It is estimated that ~90% of patients with advanced cancers die as a result of complications associated with tumor cell invasion into normal tissue and metastatic spread from primary tumor sites to secondary locations in the body, including lung, liver, bone, brain and other organs.
- Hepatocellular carcinoma (HCC), the first disease IVMT will target, is the most common form of liver cancer in adults. Liver cancer has more than tripled since 1980, cancer death rates have increased by almost 3% per year since 2000 and occurs more often in men than in women. The 5-year relative survival rate for HCC and intrahepatic bile duct cancer is 31% for localized disease, 11% for regional disease and 2% for distant disease- with SEER stages combined of 18%. Current therapies include surgery, radiation, chemotherapy, targeted therapeutic drugs (sorafenib, lenvatinib, regorafenib and cabozantinib) and immunotherapeutic drugs.
- About 42,220 new cases (30,610 in men and 11,610 in women will be diagnosed and 30,200 people (20,540 men and 9,660 women) will die of HCC in 2019.
Differentiation
- By targeting mechanisms of motility that lead to disease spread, synergizing with two standard-of-care HCC therapies (radiation and sorafenib) and inhibiting tumor angiogenesis, PDZ1i has promise to significantly improve survival.
- Since PDZ1i also modulates the cancer “stemness” and stem cell survival, responses of patients would also be expected to be durable.
- Small molecule drugs offer significant advantages in terms of use and cost improvements over cell-therapy based disease approaches.
Asset Profile & Development Plan
- Potency and duration of action: potential anti-cancer and anti-metastatic effects of PDZ1i in several animal models as well as a favorable half-life in animals supports using daily or weekly dosing in humans.
- Proof-of-concept: PDZ1i is efficacious in multiple metastatic cancer models, including liver, prevents metastatic spread of cancers after surgery and the combination of PDZ1i and sorafenib (FDA-approved drug for HCC) display profound activity against HCC in vivo in preclinical animal models containing human HCC xenotransplants.
- Safety and tolerability: No remarkable safety concerns in ADME, genotoxicity or safety pharmacology. PDZ1i exhibits an extended half-life (over 10 hours) in mice and greater than 90% bioavailability. Synthesis of PDZ1i is well established and its formulation is standard.
- Intellectual property: IVMT has exclusive licenses of the portfolio around PDZ1i patents, patent application sand know-how, as well as product-specific and MDA-9/Syntenin intellectual property. Additional PDZ inhibitors being developed in the pipeline.
- Clinical & development plan: IND enabling toxicology design has been discussed and awaits funding as do multi-institutional Phase 1 clinical trials.


