DotBio
DotBio

DotBio
Company Overview
Innovation: Multi-functional antibody therapeutics – Synergistic blockade of multiple immunosuppressive mechanisms in tumors.
Targeted Cancer(s): Tumors with immuno-suppressed microenvironment – Triple-negative breast cancer, non small cell lung cancer.
Leadership: Prof. Pär Nordlund (Scientific Founder) and Dr. Ignacio Asial (Founder and CEO). Its Advisory Board includes Prof. Bertil Lindmark, Dr. Dineli Wickramasinghe, Dr. Harry Lam and Dr. Zung Thai. (Click here to view our team)
Stage of Business: Three multi-functional therapeutic candidates currently validated in animal efficacy studies, IND-development under preparation.
Opportunity
Unmet Medical Need
Cancer is the second leading cause of death worldwide, with 18 million new cases in 2018 and 9.6 million deaths. Breast cancer is the leading cause of cancer mortality among women, while lung cancer leads for men. Recent breakthroughs in reactivating the tumor immune response via check-point blockade inhibitors, for example, have led to dramatic long-term responses for some cancer types, but immunotherapies are still not effective for most advanced cancers. A key reason is that tumor cells can overexpress and release a multitude of signaling molecules that reshape the behavior of immune cells in the tumor microenvironment. Multi-functional antibody therapies have the potential to very significantly expand the horizon for immunotherapies in cancer by providing a more refined control of the tumor immune response.
Differentiation
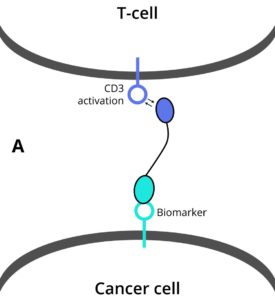
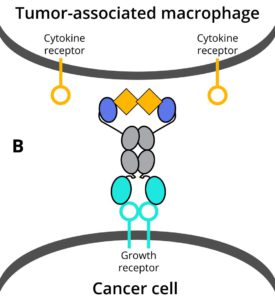
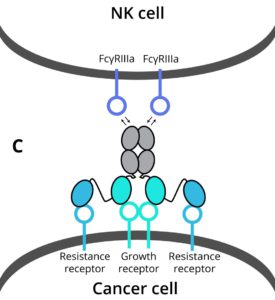
Making multi-functional antibodies is challenging and currently requires a very comprehensive protein engineering effort. This leads to few multi-functional antibody therapies being tested in preclinical studies, which then endure long development cycles before new therapies reach patients. DotBio has developed a modular and combinatorial approach to make multi-functional antibodies, where pre-made building blocks – each targeting a key protein in the tumor and its microenvironment – can be readily connected to generate multi-functional antibodies. This is made possible by DotBio’s proprietary CoFi stabilization and DotBody antibody technologies, which serve as the basis for DotBio’s multi-functional antibody discovery platform.
Using this unique approach, hundreds of prototype multi-functional antibodies can be generated in parallel and tested pre-clinically. This allows for the rapid identification of optimal therapeutic candidates with improved immunostimulatory properties while minimizing risks in the subsequent clinical trials. Additionally, by simultaneously testing many alternative multi-functional antibodies, DotBio is generating a knowledge database with new insights on the interplay between different mechanisms involved in the reshaping of the tumor immune response. This can drive the discovery of further improved multi-functional antibody therapies.
Asset Profile & Development Plan
Intellectual property: Exclusive worldwide licenses for the CoFi stabilization and DotBody antibody technologies and their related patents and patent applications, as well as proprietary phage display DotBody libraries. Additional intellectual property under development includes three multifunctional antibodies validated in animal models, as well as several additional antibodies in early-stage development.
Antibody Assets:
- DB002 is a bi-functional antibody targeting immunosuppressive receptors PDL1 (overexpressed by certain tumors) and CTLA4 (overexpressed by immune regulatory cells), with a unique mode of action. It shows efficacy in non-small cell lung cancer tumor xenograft animal models and favourable pharmacokinetics in animals.
- DB003 and DB005 target PDL1 as well as different innovative cytokines that are linked to resistance by preventing immune reactivation. They show efficacy in triple-negative breast cancer and non-small cell lung cancer tumor xenograft models in animals. They are currently undergoing additional efficacy testing against diverse tumor types.
Clinical development plans: the goal is to pursue at least one of these therapeutic candidates towards IND-filing and Phase I clinical trials, while other assets continue their pre-clinical development. IND-enabling studies (process development, large-animal toxicology and GMP manufacturing) are planned and awaiting further funding. Partnerships with public and private medical institutions could help accelerate the clinical development of these candidates, to bring therapeutic benefits to patients that urgently need them.

